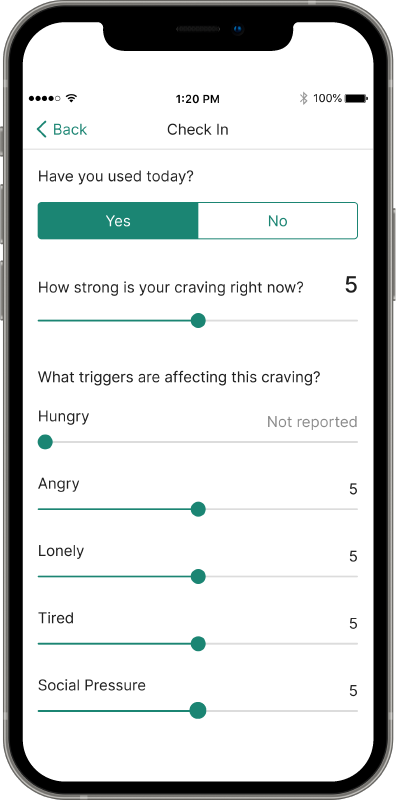
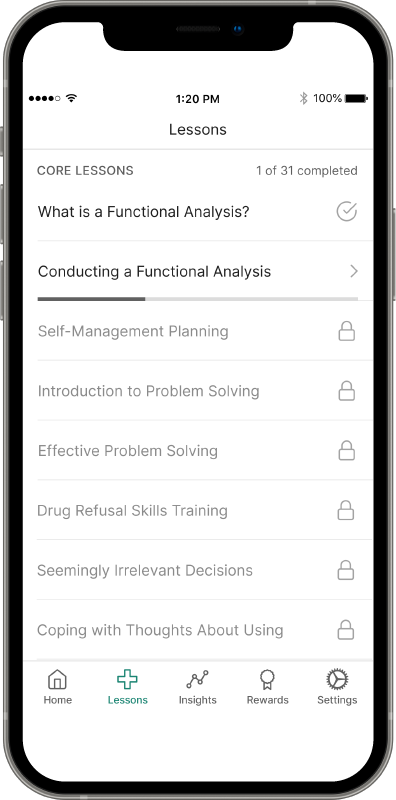
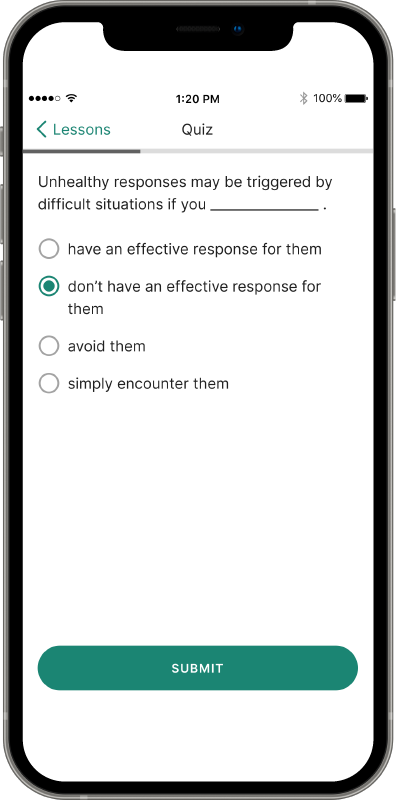
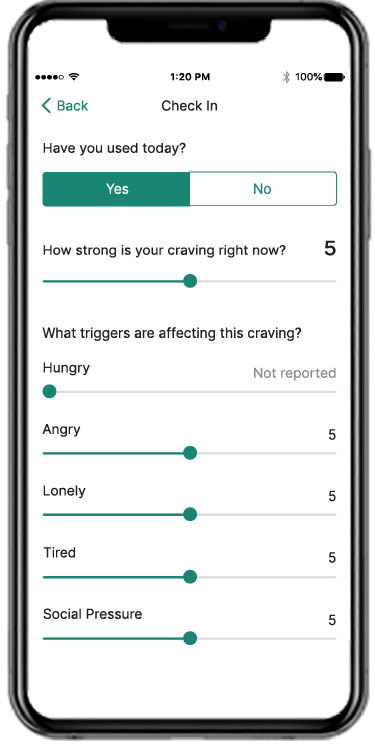
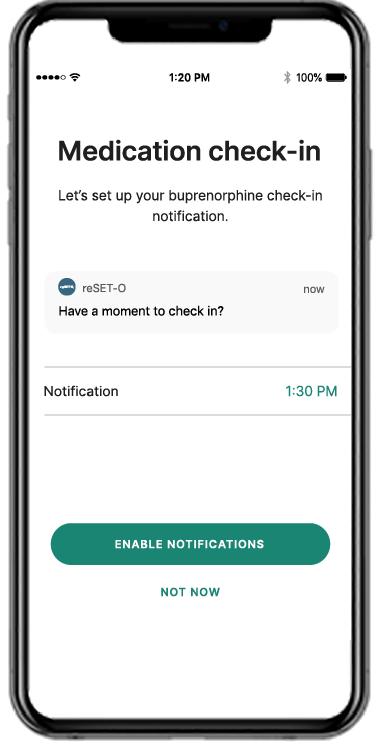
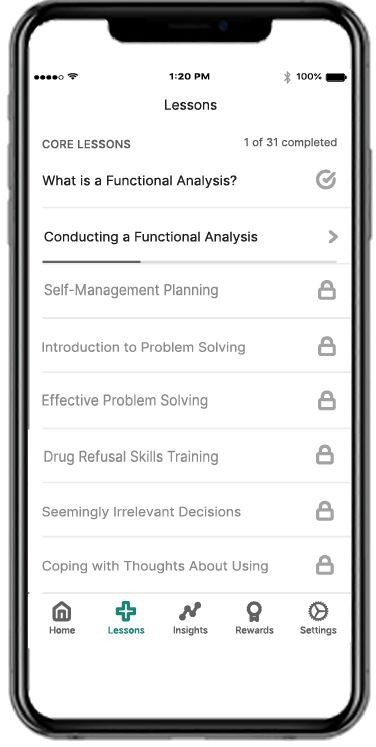
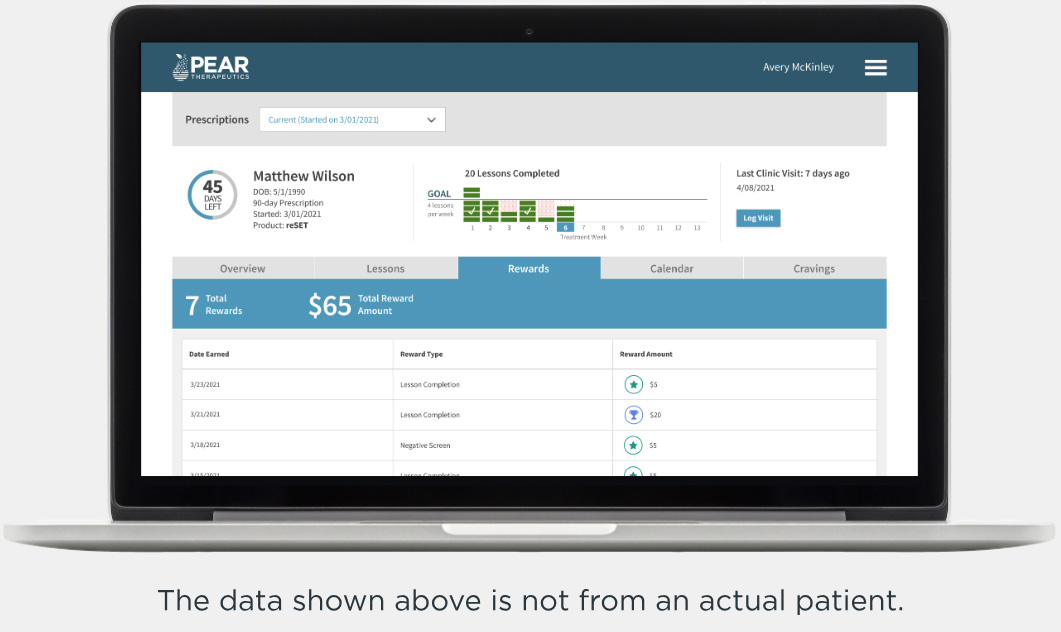
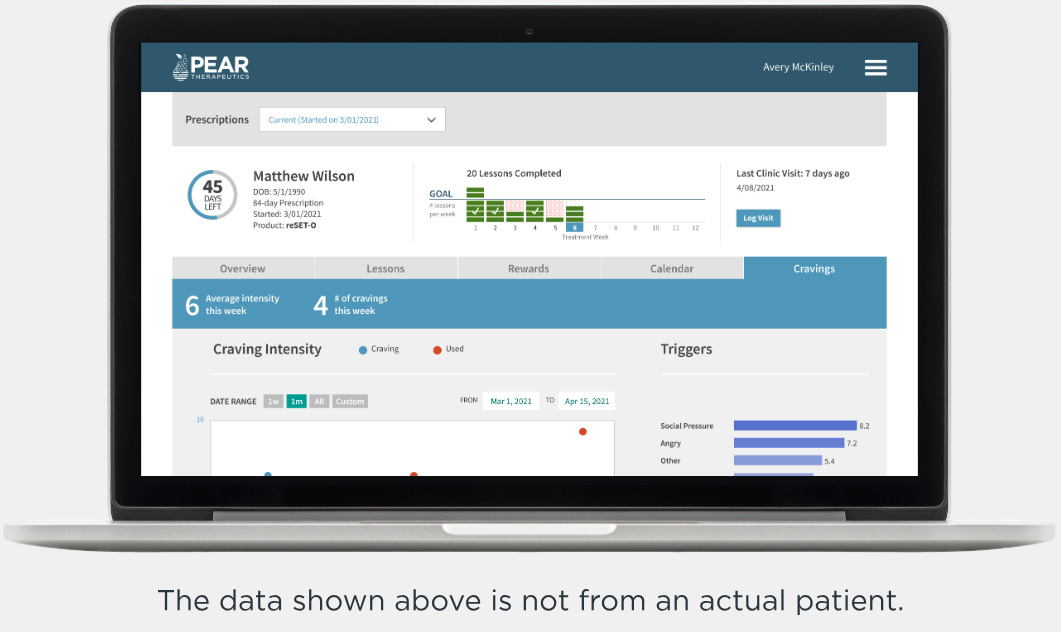
Recovery is a round-the-clock process, and patients can’t always see you when triggers and cravings arise. RESET® and RESET-O® give you 24/7 support on your patients' smartphone.1,2*
Put the power of a 24/7 tool in patients’ hands
Provide anytime, anywhere access to evidence-based approaches, like cognitive behavioral therapy, fluency training, and contingency management, with the clinically proven prescription digital therapeutic that meets their needs.4,5
RESET
 A 12-week clinical study showed that patients using reSET were more than 2x more likely to abstain from substance use than those who didn’t use RESET.5*
A 12-week clinical study showed that patients using reSET were more than 2x more likely to abstain from substance use than those who didn’t use RESET.5**During the last 4 weeks of trial.
IN A 12-WEEK CLINICAL STUDY OF PEOPLE WITH SUD
Patients who used RESET in outpatient treatment were 2 times more likely to abstain from substance use than those who didn’t use RESET.1Treatment with RESET did not demonstrate a significant difference in unanticipated adverse events compared with treatment as usual.3
RESET-O

*During 12 weeks of treatment. Those who didn’t add reSET-O received treatment as usual.
For individuals with opioid use disorder, RESET-O® is the only FDA-authorized prescription digital therapeutic that’s proven to help patients stay in treatment longer.4,7 It works in a way that meets patients where they already are, on their smartphones, so they can turn screen time into therapy time. Patients prescribed RESET-O download it to their phone for secure, discreet, and convenient access to therapy, interactive learning, and support.
IN A 12-WEEK CLINICAL STUDY OF PEOPLE WITH OUD WHO STAYED IN TREATMENT2
Of people who added RESET-O to their buprenorphine stayed in treatment
vs.
Who didn’t add RESET-O to their buprenorphine stayed in treatment
The observed adverse events were of a type and frequency to be anticipated in a large population of patients with OUD or associated with buprenorphine pharmacotherapy, particularly during the induction phase.2
The adverse events observed were not determined to be device related.2
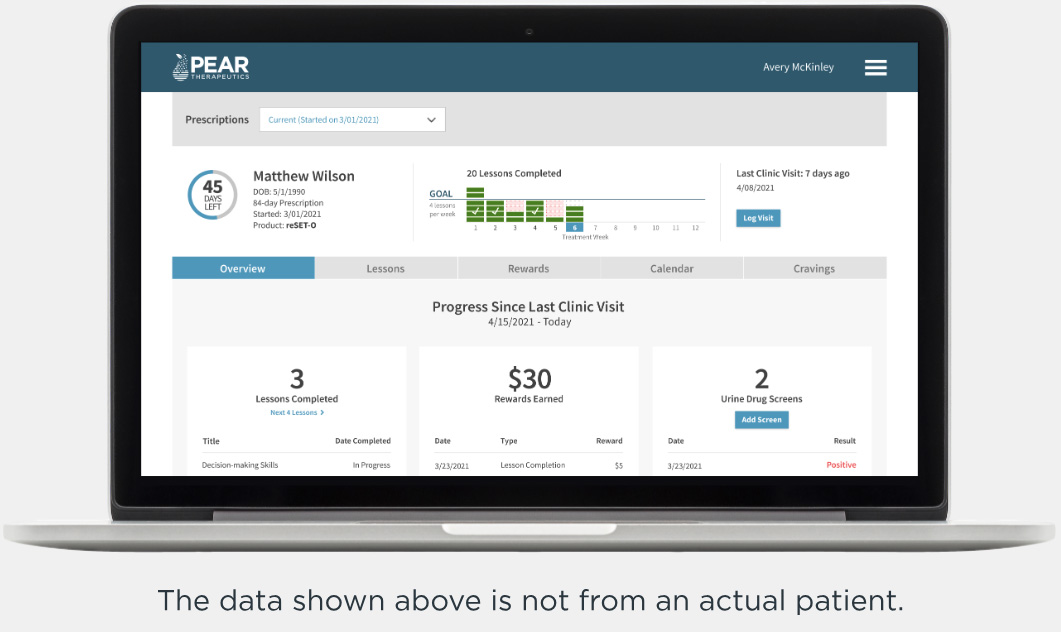
Clinician Dashboard
Optimize treatment and enrich sessions using real-world patient data4,5
RESET® Indications for Use:
RESET is intended to provide cognitive behavioral therapy, as an adjunct to a contingency management system, for patients 18 years of age and older, who are currently enrolled in outpatient treatment under the supervision of a clinician. reSET is indicated as a 12-week (90 day) prescription-only treatment for patients with substance use disorder (SUD), who are not currently on opioid replacement therapy, who do not abuse alcohol solely, or who do not abuse opioids as their primary substance of abuse.
It is intended to:
- increase abstinence from a patient’s substances of abuse during treatment, and
- increase retention in the outpatient treatment program.
RESET® Important Safety Information for Patients:
Warnings/precautions: Do not use RESET to communicate any emergency, urgent or critical information. RESET does not include features that can send alerts or warnings to your clinician. If you have feelings or thoughts of harming yourself or others, please dial 911 or go to the nearest emergency room.
RESET is intended for patients whose primary language is English with a reading level of 7th grade or above, who have access to an Android/iOS tablet or smartphone, and who are familiar with the use of smartphone applications (apps). You should be able to upload data periodically, i.e. have internet/wireless connection access.
RESET is not intended to be used as stand-alone therapy for substance use disorder (SUD) and does not replace care by your provider or outpatient treatment. RESET is not a substitute for your medications. You should continue to take your medications as directed by your provider.
The long-term benefit of treatment with RESET on abstinence has not been evaluated in studies lasting beyond 12 weeks (90 days) in the SUD population. The ability of RESET to prevent potential relapse after treatment discontinuation has not been studied.
The safety and effectiveness of RESET has not been established in patients enrolled in opioid treatment programs reporting opioids as their primary substance of abuse.
RESET-O® Indications for Use:
RESET-O prescription digital therapeutic is a 12-week (84 day) software application intended to increase retention of patients with opioid use disorder (OUD) in outpatient treatment by providing cognitive behavioral therapy, as an adjunct to outpatient treatment that includes transmucosal buprenorphine and contingency management, for patients 18 years or older who are currently under the supervision of a clinician. reSET-O is indicated as a prescription-only digital therapeutic.
RESET-O® Important Safety Information for Patients:
Warnings/precautions: Do not use RESET-O to communicate any emergency, urgent or critical information. RESET-O does not include features that can send alerts or warnings to your clinician. If you have feelings or thoughts of harming yourself or others, please dial 911 or go to the nearest emergency room.
RESET-O is intended for patients whose primary language is English with a reading level of 7th grade or above, who have access to an Android/iOS tablet or smartphone, and who are familiar with the use of smartphone applications (apps). You should be able to upload data periodically, i.e. have internet/wireless connection access.
RESET-O is not intended to be used as stand-alone therapy for Opioid Use Disorder (OUD) and does not replace care by your provider or outpatient treatment. RESET-O is not a substitute for your medications. You should continue to take your medications as directed by your provider.
The long-term benefit of RESET-O has not been evaluated in studies lasting beyond 12 weeks (84 days) in the OUD population. The ability of reSET-O to prevent potential relapse after therapy discontinuation has not been studied.
References:
1. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. US Department of Health and Human Services; 2021. HHS publication no. PEP21-07-01-003, NSDUH Series H-56. Accessed April 15, 2022.
https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
2. Medication-assisted treatment (MAT). Substance Abuse and Mental Health Services Administration. Updated March 30, 2022. Accessed April 15, 2022.
https://www.samhsa.gov/medication-assisted-treatment
3. Data on file. Pear Therapeutics. 2021
4. RESET-O Clinician directions for use. Pear Therapeutics, Inc. 2020.
5. RESET Clinician directions for use. Pear Therapeutics, Inc. 2020.
6. FDA permits marketing of mobile medical application for substance use disorder. News Release. Silver Spring, MD: US Food and Drug Administration. September 14, 2017. Accessed May 18, 2020. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-mobile-medical-application-substance-use-disorder
7. FDA clears mobile medical app to help those with opioid use disorder stay in recovery programs. News release. Silver Spring, MD: US Food and Drug Administration. December 10, 2018. Accessed May 18, 2020. https://www.fda.gov/news-events/press-announcements/fda-clears-mobile-medical-app-help-those-opioid-use-disorder-stay-recovery-programs